Cell Extraction-Free 1-Step RT-qPCR Kit (Low ROX)
SKU: CRTQ02-01
Cell Extraction-Free 1-Step RT-qPCR Kit (Low ROX)
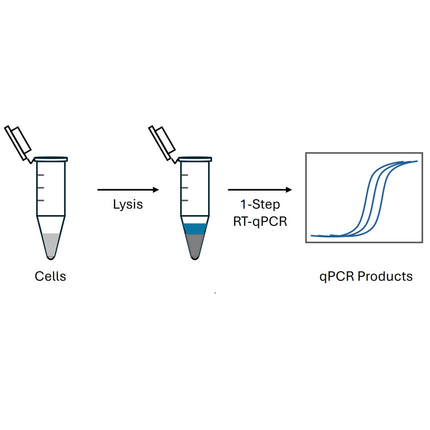
Intended Use The Master Mix is used for real-time qualitative and quantitative RT-PCR amplifications with SYBR Green dye for extraction-free... Read more
$180.00
Want a Bulk Order? Contact us!
Our team is available Monday through Friday from 9:00am-5:30pm PST.